Cardiac Electrophysiology and Tension Development Software
The cardiac simulation tool acCELLerate is written (C++) and maintained by the Institute of Biomedical Engineering of the KIT. acCELLerate is the replacement of the formerly used tool xCELLent. It´s functionality ranges from simulation of cardiac electrophysiology to muscle tension development, for small isotropic tissue patches as well as complex whole heart simulation. Due to the modular structure, different methods can be used and various parameters can be set, e.g. simulation with several cell models in one dataset is feasible.
The implementation of acCELLerate is time and memory efficient by the use of PETSc for parallelization and numerical solving. Another advantage of PETSc is the possibility to choose several solvers and preconditioners at runtime which can be used for individual acceleration of the simulation.
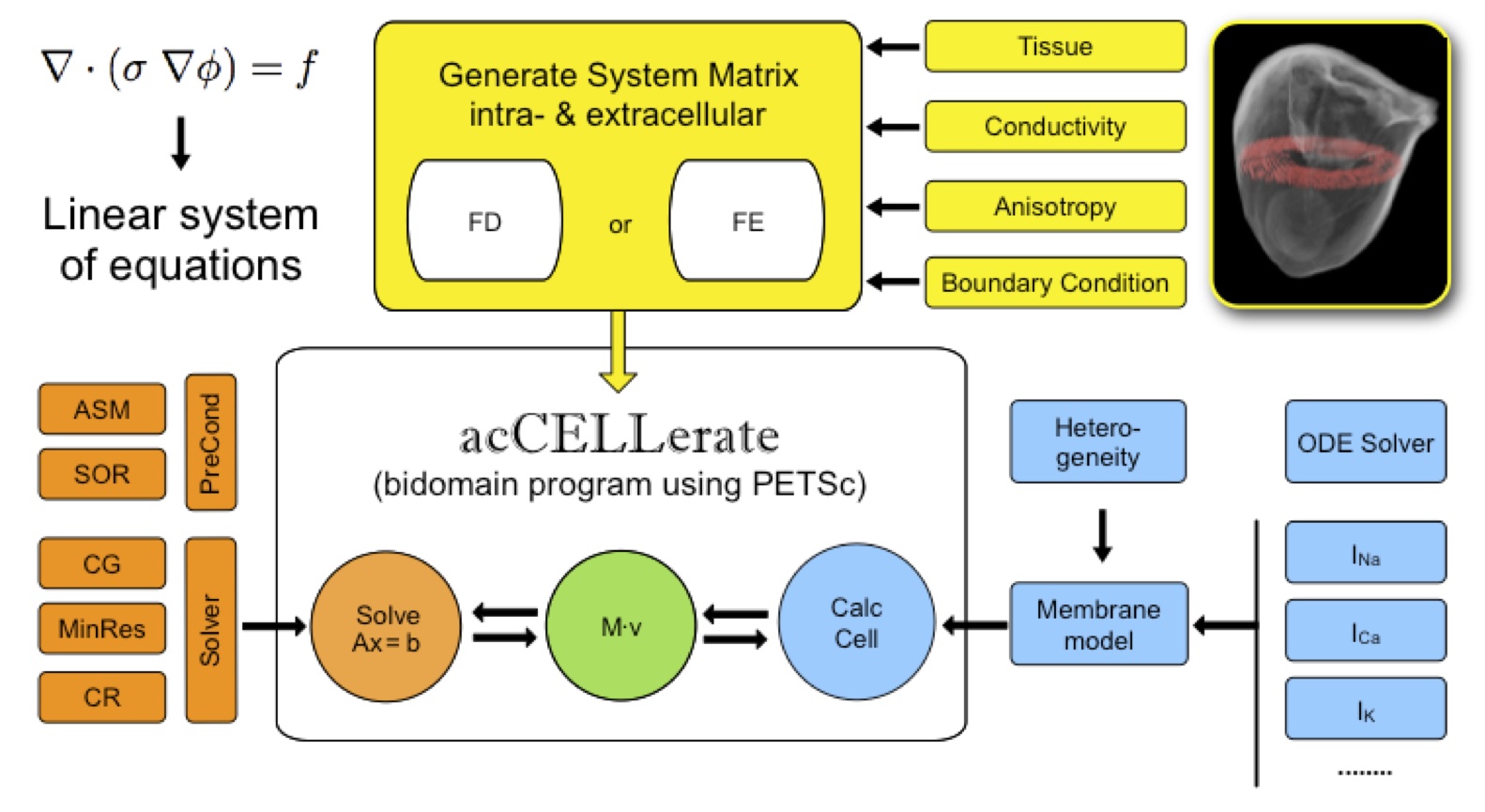
Fig. 1: Modular structure of acCELLerate (bidomain computation).
The modular structure of acCELLerate is depicted schematically in figure 1. Inputs of the tool are the geometrical dataset, e.g. the heart of the Visible Man including tissue classes, fiber orientation and heterogeneity. The matrix generation method can be chosen to be either Finite Difference (FD) or (cell-centered) Finite Element (FE) method. Currently, the matrix generation is limited to cubic grids. Various cell models for both humans and mammals are available to describe the appropriate tissue properties. Monodomain, bidomain and multi-domain equations are implemented to simulate cardiac excitation conduction as accurate as possible (see fig. 2). This and also the tension development distribution of the cardiac muscle can be calculated for every time step of the cardiac cycle.
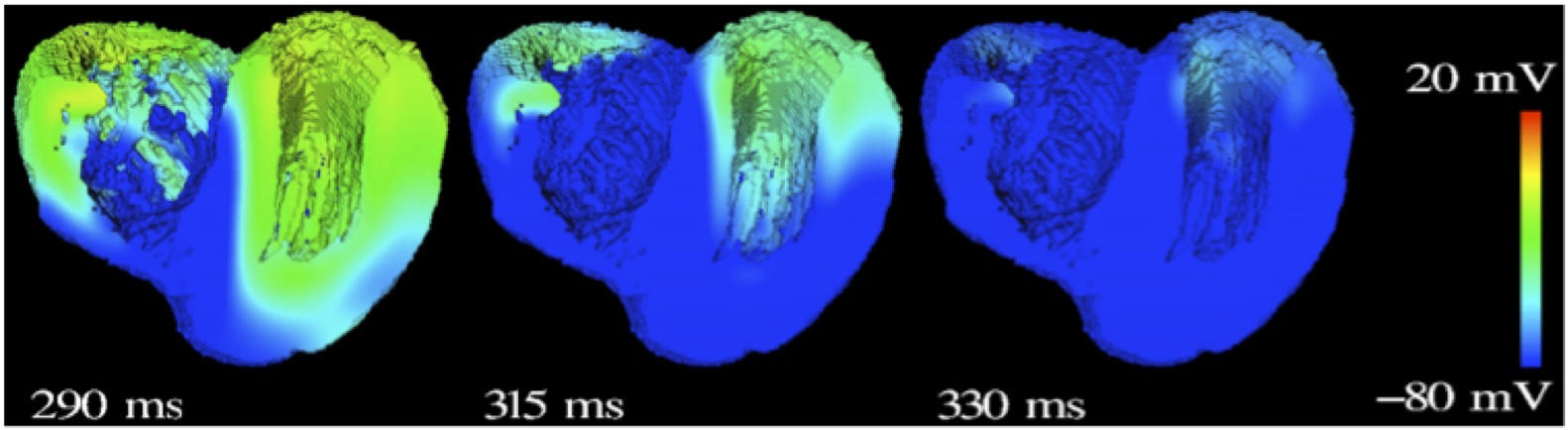
Fig. 2: Repolarization of the human ventricle, excitation conduction computed by acCELLerate.
Not only the normal behavior of a healthy heart can be simulated, but also several pathologies, e.g. atrial fibrillation or ischemia (see fig. 3). Furthermore, the influence of drugs can be investigated. This makes acCELLerate a very powerful instrument for research in cardiology.
Further software packages of the Institute of Biomedical Engineering of KIT allow the segmentation of individual MRI data, the simulation of body ECGs or mechanical contraction of the heart.
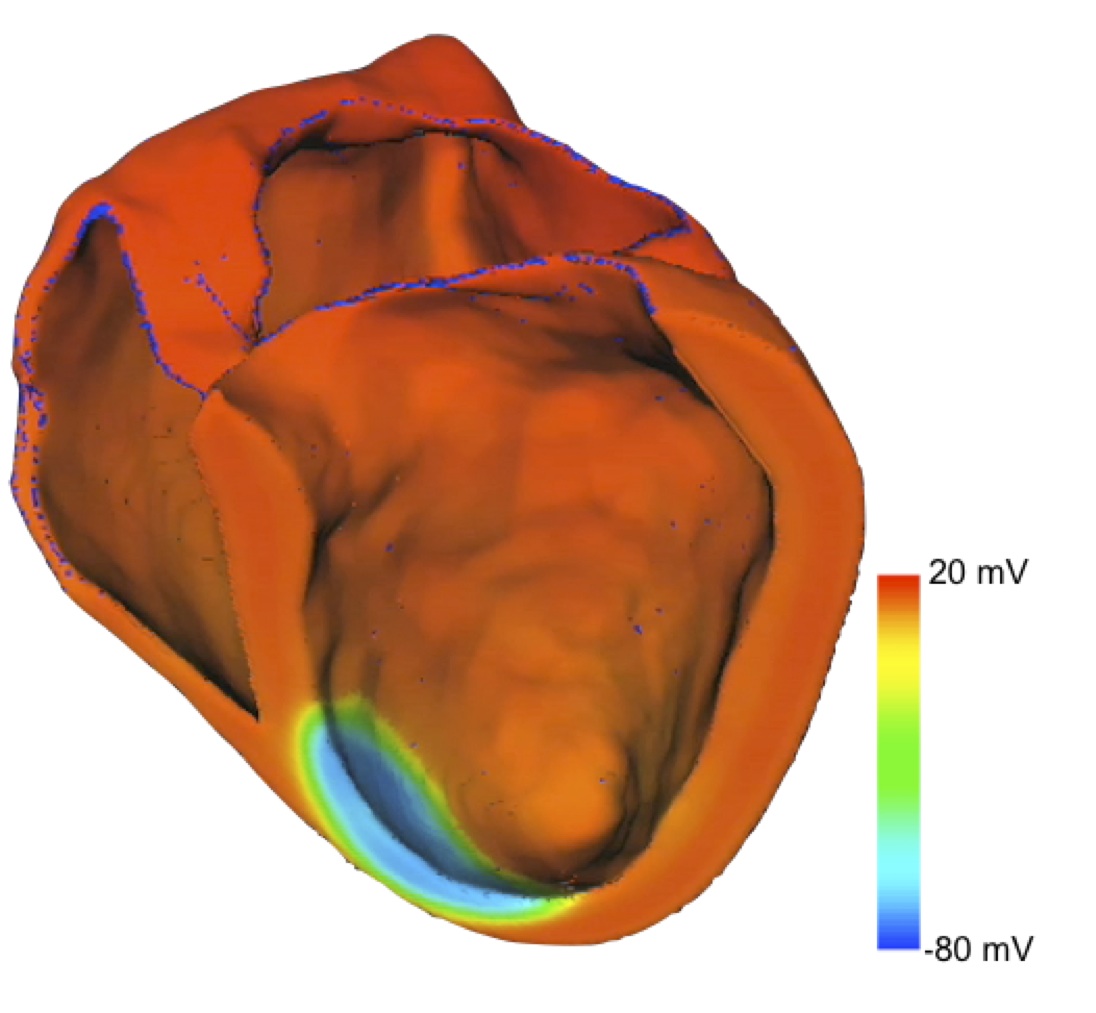
Fig. 3: Electrical excitation with ischemic tissue near apex.
For interest in joining to use or further develop acCELLerate please contact Axel Loewe.
In the following journal publications acCELLerate was used in order to generate results:
- S Pollnow, J Greiner, T Oesterlein, EM Wülfers, O Dössel. Mini Electrodes on Ablation Catheters: Valuable Addition or Redundant Information?—Insights from a Computational Study. Computational and Mathematical Methods in Medicine, vol. 2017 (Article ID 168629), pp.13, 2017
- T Oesterlein, D Frisch, A Loewe, G Seemann, C Schmitt, O Dössel, A Luik, Basket-Type Catheters: Diagnostic Pitfalls caused by Deformation and Limited Coverage. BioMed Research International (Article ID 5340574) , pp. 1-13, 2016
- A Loewe, MW Krueger, F Holmqvist, O Dössel, G Seemann, PG Platonov. Influence of the earliest right atrial activation site and its proximity to interatrial connections on P-wave morphology.
Europace, vol. 18, pp. iv35-iv43, 2016 - A Loewe, M Wilhelms, J Schmid, MJ Krause, F Fischer, D Thomas, EP Scholz, O Dössel, G Seemann. Parameter estimation of ion current formulations requires hybrid optimization approach to be both accurate and reliable. Frontiers in Bioengineering and Biotechnology, vol. 3, pp. 209, 2016
- C Lenk, FM Weber, M Bauer, M Einax, P Maass, G Seemann. Initiation of atrial fibrillation by interaction of pacemakers with geometrical constraints. Journal of Theoretical Biology, vol. 366, pp. 13-23, 2015
- A Loewe, M Wilhelms, F Fischer, EP Scholz, O Doessel, G Seemann. Arrhythmic potency of human ether-a-go-go-related gene mutations L532P and N588K in a computational model of human atrial myocytes. Europace, vol. 16(3), pp. 435-443, 2014
- M Keller, S Schuler, M Wilhelms, G Lenis, G Seemann, C Schmitt, O Doessel, A Luik. Characterization of Radiofrequency Ablation Lesion Development Based on Simulated and Measured Intracardiac Electrograms. IEEE Trans. Biomed. Engin., vol. 61(9), pp. 2467-2478, 2014
- MW Krueger, G Seemann, KS Rhode, DUJ Keller, C Schilling, A Arujuna, J Gill, MD O'Neill, R Razavi, O Dössel. Personalization of Atrial Anatomy and Elelectophysiology as a Basis for Clinical Modeling of Radio-Frequency-Ablation of Atrial Fibrillation. IEEE Trans. Med. Imag., vol. 32(1), pp. 73-84, 2013
- MW Krueger, WHW Schulze, KS Rhode, R Razavi, G Seemann, O Dössel. Towards personalized clinical in-silico modeling of atrial anatomy and electrophysiology. Med. Biol. Engin. Comput., vol. 51(11), pp. 1251-1260, 2013
- MW Krueger, A Dorn, DUJ Keller, F Holmqvist, J Carlson, PG Platonov, KS Rhode, R Razavi, G Seemann, O Dössel. In-silico modeling of atrial repolarization in normal and atrial fibrillation remodeled state. Med. Biol. Engin. Comput., vol. 51(10), pp. 1105-1119, 2013
- EP Scholz, P Carrillo-Bustamante, F Fischer, M Wilhelms, E Zitron, O Dössel, HA Katus, G Seemann. Rotor termination is critically dependent on kinetic properties of I kur inhibitors in an in silico model of chronic atrial fibrillation. PloS one, vol. 8(12), pp. e83179, 2013
- M Wilhelms, H Hettmann, MMC Maleckar, JT Koivumäki, O Dössel, G Seemann. Benchmarking electrophysiological models of human atrial myocytes. Front Physiol., vol. 3(487), pp. 1-16, 2013
- M Burdumy, A Luik, P Neher, R Hanna, MW Krueger, C Schilling, H Barschdorf, C Lorenz, G Seemann, C Schmitt, O Dössel, FM Weber. Comparing measured and simulated wave directions in the left atrium - a workflow for model personalization and validation. Biomed. Tech. (Berl), vol. 57(2), pp. 79-87, 2012
- O Dössel, MW Krueger, FM Weber, M Wilhelms, G Seemann. Computational modeling of the human atrial anatomy and electrophysiology. Med. Biol. Engin. Comput., vol. 50(8), pp. 773-799, 2012
- DUJ Keller, DL Weiss, O Dössel, G Seemann. Influence of IKs heterogeneities on the genesis of the T-wave: a computational evaluation. IEEE Trans. Biomed. Engin., vol. 59(2), pp. 311-322, 2012
- M Wilhelms, C Rombach, EP Scholz, O Doessel, G Seemann. Impact of amiodarone and cisapride on simulated human ventricular electrophysiology and electrocardiograms. Europace, vol. 14, pp. v90-v96, 2012
- DUJ Keller, O Jarrousse, T Fritz, S Ley, O Dössel, G Seemann. Impact of physiological ventricular deformation on the morphology of the T-wave: a hybrid, static-dynamic approach. IEEE Trans. Biomed. Engin., vol. 58(7), pp. 2109-2119, 2011
- SA Niederer, E Kerfoot, AP Benson, MO Bernabeu, O Bernus, C Bradley, EM Cherry, R Clayton, FH Fenton, A Garny, E Heidenreich, S Land, M Maleckar, P Pathmanathan, G Plank, JF Rodriguez, I Roy, FB Sachse, G Seemann, O Skavhaug, NP Smith. Verification of cardiac tissue electrophysiology simulators using an N-version benchmark. Philosophical Transactions of the Royal Society A, vol. 369(1954), pp. 4331-4351, 2011
- FM Weber, DUJ Keller, S Bauer, G Seemann, C Lorenz, O Dössel. Predicting tissue conductivity influences on body surface potentials-an efficient approach based on principal component analysis. IEEE Trans. Biomed. Engin., vol. 58(2) , pp. 265-273, 2011
- FM Weber, A Luik, C Schilling, G Seemann, MW Krueger, C Lorenz, C Schmitt, O Dössel. Conduction velocity restitution of the human atrium--an efficient measurement protocol for clinical electrophysiological studies. In IEEE Trans. Biomed. Engin., vol. 58(9), pp. 2648-2655, 2011
- M Wilhelms, O Dössel, G Seemann. In silico investigation of electrically silent acute cardiac ischemia in the human ventricles. IEEE Trans. Biomed. Engin., vol. 58(10), pp. 2961-2964, 2011
- MW Krueger, S Severi, K Rhode, S Genovesi, FW Weber, A Vincenti, P Fabbrini, G Seemann, R Razavi, O Dössel. Alterations of atrial electrophysiology related to hemodialysis session: insights from a multiscale computer model. J. Electrocard., vol. 44(2) , pp. 176-183, 2011
- DUJ Keller, FM Weber, G Seemann, O Dössel. Ranking the Influence of Tissue Conductivities on Forward-Calculated ECGs. IEEE Trans. Biomed. Engin., vol. 57, pp. 1568 - 1576, 2010
- G Seemann, FB Sachse, M Karl, DL Weiss, V Heuveline, O Dössel. Framework for modular, flexible and efficient solving the cardiac bidomain equation using PETSc. Progr. Industr. Math., vol. 15, pp. 363-369, 2010
- G Seemann, DUJ Keller, MW Krueger, FM Weber, M Wilhelms, O Dössel. Electrophysiological Modeling for Cardiology: Methods and Potential Applications. Information Technology, vol. 52, pp. 242-249, 2010
- FM Weber, C Schilling, G Seemann, A Luik, C Schmitt, C Lorenz, O Dössel. Wave Direction and Conduction Velocity Analysis from Intracardiac Electrograms - A Single-Shot Technique. IEEE Trans. Biomed. Engin., vol. 57, pp. 2394-2401, 2010
- DL Weiss, M Ifland, FB Sachse, G Seemann, O Dössel. Modeling of Cardiac Ischemia in Human Myocytes and Tissue including Spatiotemporal Electrophysiological Variations. Biomedizinische Technik, vol. 54, pp. 107-125, 2009
- FB Sachse, AP Moreno, G Seemann, JA Abildskov. A model of electrical conduction in cardiac tissue including fibroblasts. Ann. Biomed. Engin., vol. 37, pp. 874-889, 2009