Estimating Mesh Resolution for EMI Model Simulations
- chair:Computational Cardiac Modeling
- type:Bachelor thesis
- tutor:
-
Introduction.
The heart functions as an excitable medium, where electrical excitation waves propagate through cardiac tissue to trigger muscle contraction. Cardiac arrhythmias arise from
abnormal wave propagation, and understanding the mechanisms underlying different wave patterns is crucial. Computational models play a key role in this endeavour, offering insights into excitation dynamics by simulating the heart’s electrophysiology with both spatial and temporal discretisation. Most state-of-the-art simulations employ meshes that effectively average over several hundred myocytes. However, recent studies indicate that electrophysiological processes at the sub-cellular scale can critically influence the initiation of arrhythmias. Therefore, high-resolution models that capture microstructural details should also be considered.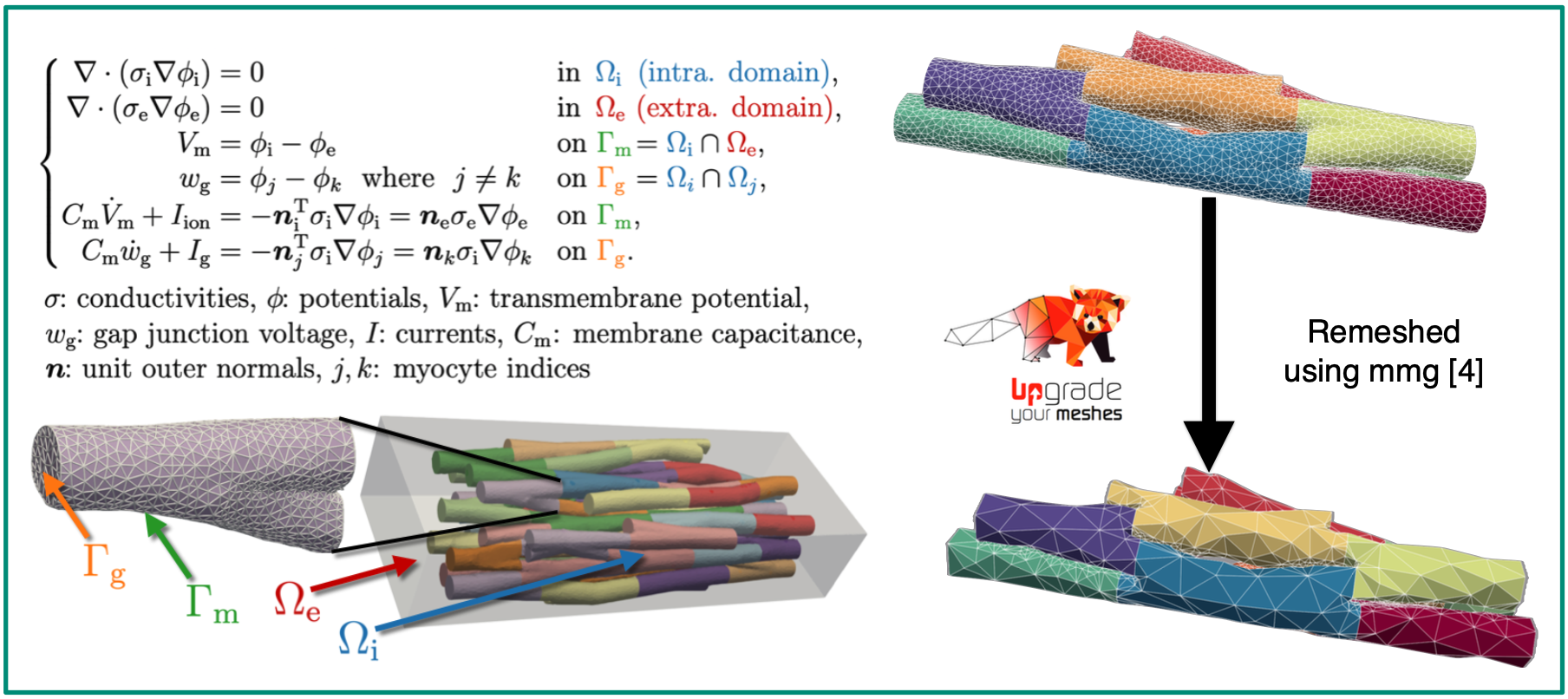
Thesis Topic.
We are working with the extracellular-membrane-intracellular (EMI) model (left image), which explicitly resolves myocytes and the surrounding extracellular space within the computational mesh to study the microstructural behaviour of excitation waves in cardiac tissue [1]. The EMI model was recently implemented in the electrophysiology simulator openCARP [2], but simulation runtimes remain a major limitation.
This project aims to reduce runtimes for EMI simulations by decreasing the mesh resolution — thereby lowering the number of degrees of freedom (DOFs) — in synthetically generated meshes [3]. To this end, we simulate wave propagation on a high-resolution EMI mesh and compute electrograms (EGMs) using a point electrode at a defined location, treating this as the reference or ground truth. We then systematically coarsen the reference mesh (right image), repeat the same simulation for each coarsened version, and calculate EGMs at the same locations. By comparing these EGMs to the reference, we aim to determine a resolution threshold beyond which electrophysiologically relevant features are lost—thus identifying the coarsest mesh that still yields accurate EGM predictions.Sources
[1] Tveito A, Mardal KA, Rognes ME. Modeling Excitable Tissue: The EMI Framework. Simula Springer Briefs on
Computing. Springer International Publishing; 2020.
[2] https://opencarp.org/
[3] Potse M, Cirrottola L, Froehly A. A practical algorithm to build geometric models of cardiac muscle structure. In:
8th European Congress on Computational Methods in Applied Sciences and Engineering (ECCOMAS). Oslo,
Norway; 2022.
[4] Mmg version 5.8.0 https://github.com/MmgTools/mmgThis work was supported by the European High- Performance Computing Joint Undertaking EuroHPC under grant agreement No 955495 (MICROCARD) cofunded by the Horizon 2020 programme of the European Union (EU), the German Federal Ministry of Education and Research and the French National Research Agency ANR.

